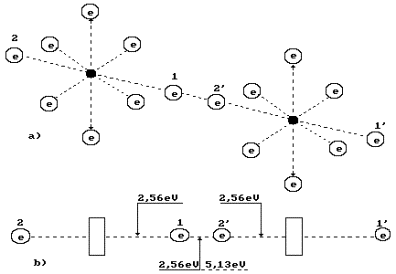
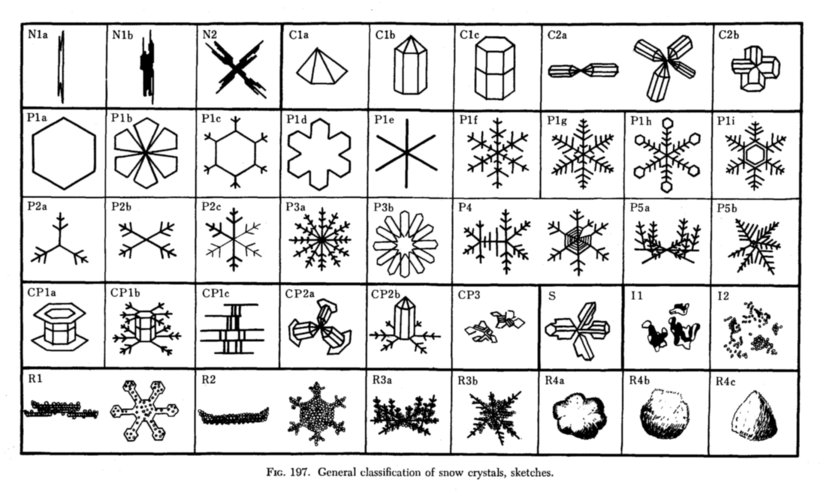
Diagram of binding energy distribution between the electrons in the oxygen molecule
http://www.guns.connect.fi/innoplaza/energy/story/Kanarev/ozone/index.html
The Geometry of Snowflakes - Oxygen Structure by Sneelock
http://www.dailykos.com/story/2009/11/15/804812/-The-Geometry-of-SnowflakesOxygen-Structure
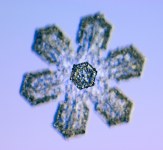
One of the most amazing and plentiful phenomenons in nature, is a snowflake. Formed in a freezing atmosphere of free water molecules, which lock themselves together in a hexagonally based crystal structure that is delicate and nearly perfectly balanced. What mechanism creates such a structure? Why do freezing water molecules arrange themselves so easily and consistently at the 120 degree angle that forms this hexagonal structure? Our science does not yet provide a good explanation for that. Ice forms in lots of other configurations too. And the angles involved in those await explanation as well.http://www.dailykos.com/story/2009/11/15/804812/-The-Geometry-of-SnowflakesOxygen-Structure
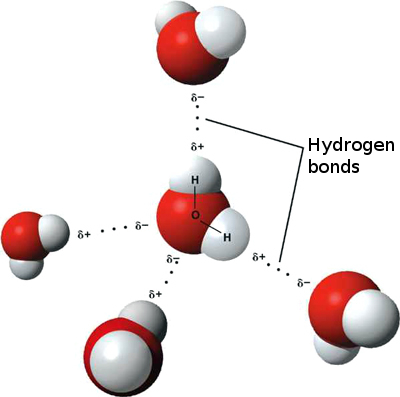
Probably the most important aspect of water -- the thing that causes most of it's unique properties -- is its electric polarity. When an Oxygen atom bonds with two Hydrogen atoms to form water, the Hydrogens always bond to the Oxygen atom at one end. This makes the hydrogen side of the molecule electro-positive, and the other side, with free electrons, electro-negative. The opposite charges of the two sides makes water molecules strongly attracted to each other -- and to many other electrically "polar" molecules, which makes water such a good solvent. This electric attraction is the reason for water's exceptionally strong surface tension. Which makes water droplets form such perfect spheres, that billions of these droplets reflect and refract sunlight so consistently that we see the whole visible spectrum of light displayed in rainbows all the time.
Like the 120 degree angle formed regularly in snowflakes, liquid or gaseous water has a consistent angular formation. With the hydrogen atoms always sharing two sets of two electrons on one side of the Oxgen nucleus. And the two Hydrogen nuclei always forming an angle of about 104.4 degrees with each other.
As I mentioned before, The geometry of the various angles in which the elements arrange themselves is not yet fully understood. There are theories about how the angles result from the attractive and repulsive forces of the electrons in atoms. But they don't work consistently across different elements. But we do have descriptions of what the angles are for most elements. And we know which elements can combine with each other. As well as the angles that form between them when they do. But we haven't understood in a convincing way, why the elements form the angles that they do. We don't have anything that explains the source of the geometry. We just know what the geometry is.
Below is a model of nuclear formation that goes beyond the current one. A model that describes the three dimensional configuration of protons and neutrons in a nucleus -- and accounts for the resulting geometry of atomic bonds that science has already determined. Oxygen is just one example of an element that this model explains the geometry of, more convincingly than the current model. I'll give a brief description of the model, then focus on the resulting configuration for Oxygen, which determines the known bonding angles that form in both water and snowflakes.
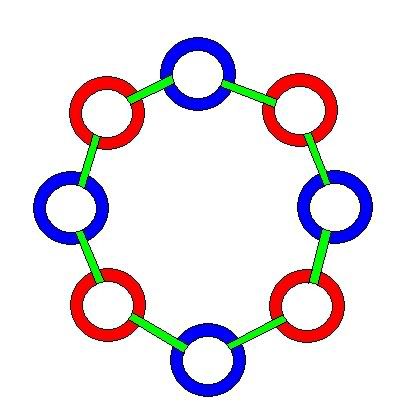
In this model, protons and neutrons are composed of different numbers of the same elements -- called Hawkrings. There are two types of Hawkrings. Left(positive) rings and right(negative) rings. A neutron consists of 4 left Hawkrings and 4 right Hawkrings, joined together in an alternating chain of 8 rings. The chain joins end to end forming a circle of 8 rings. The rings are bound together by gluons. Below is a picture of a neutron in its natural state, a circle. The shape it forms, if it's not attached to another hadron. I'll use the term hadron here to refer to either protons or neutrons, or both -- though this term generally refers to anything inside a nucleus.
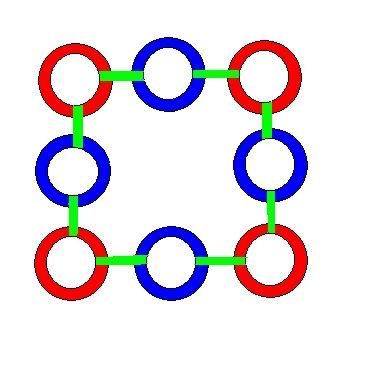
A proton is formed from the same configuration as a neutron, except that an additional left ring joins the formation in the center, bonding to the 4 right rings in the outer ring. This gives a proton a square shape, pictured below.
Neutrons are more flexible than protons. They can assume the square shape of a proton, when they are bound to other hadrons, or they can accommodate themselves to other shapes. In the spherical configuration of an Oxygen nucleus, neutrons do both of these things. A squared proton is below.
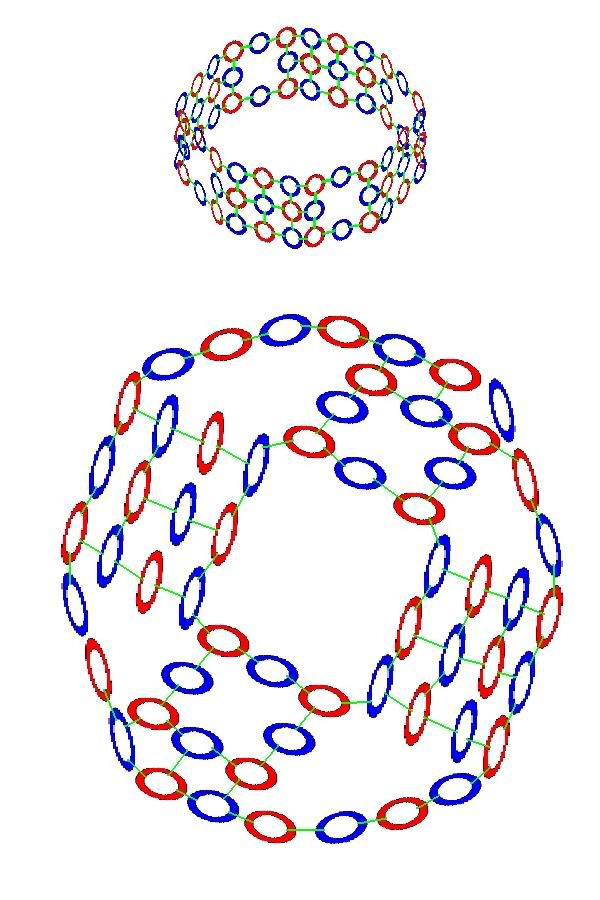
In the formation of the elements, individual Hawkrings in protons and neutrons become bonded to rings in other hadrons. By gluons, that join these building blocks together in certain shapes. The four Elements following Hydrogen, Helium(2), Lithium(3), Beryllium(4), and Boron(5), all have box-like shapes. But the next 5, Carbon(6), Oxygen(8), Nitrogen(9), Fluorine(10) and Neon(11) are all spheres. Carbon is built on a six-sided ring, with 6 more hadrons filling out the top and bottom. And the others all built on a similar eight-sided ring, with differing numbers of protons and neutrons above and below, according to their atomic number.
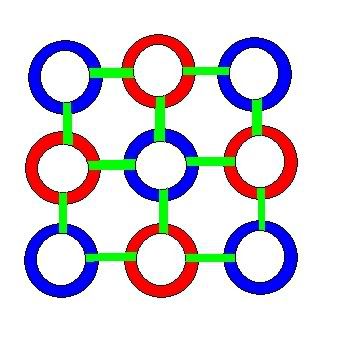
The formation of an Oxygen nucleus is pictured below. First an alternating ring of 4 protons and 4 neutrons forms a ring. On top, two protons attach to two opposing neutrons in the ring. Then two neutrons attach to the ring and to the corners of the protons. Tying the top together. And leaving the two protons facing up at almost exactly 45 degrees from horizontal. Perhaps a little bit higher than that because the neutrons are not attached to the ring as symmetrically as the protons. The bottom is the same configuration, but rotated 90 degrees relative to the top.
Oxygen(8) is the most perfectly symmetrical of all the spheres. And it's easy to see how the geometry of the Oxygen nucleus creates the amazing properties of water. The location of the proton faces in a nucleus determines where an element's electrons will be arranged. In Oxygen, The 8-ring of alternating protons and neutrons means, if you were standing in the center of the nucleus, directly in front, behind and to your right and left, there would be a proton, and an electron extending directly out from each of those. Above, would be two more protons and electrons. If you turn 45 degrees left and raise your arms up 45 degrees above horizontal, that's where the two top electrons will be. Same for the bottom, but you'd rotate 45 degrees right from your original position. So the top and bottom proton faces are oriented at 90 degrees to one another. The angles formed by these eight lines fit perfectly with what science has determined about the bonding angles in water and in snowflakes.
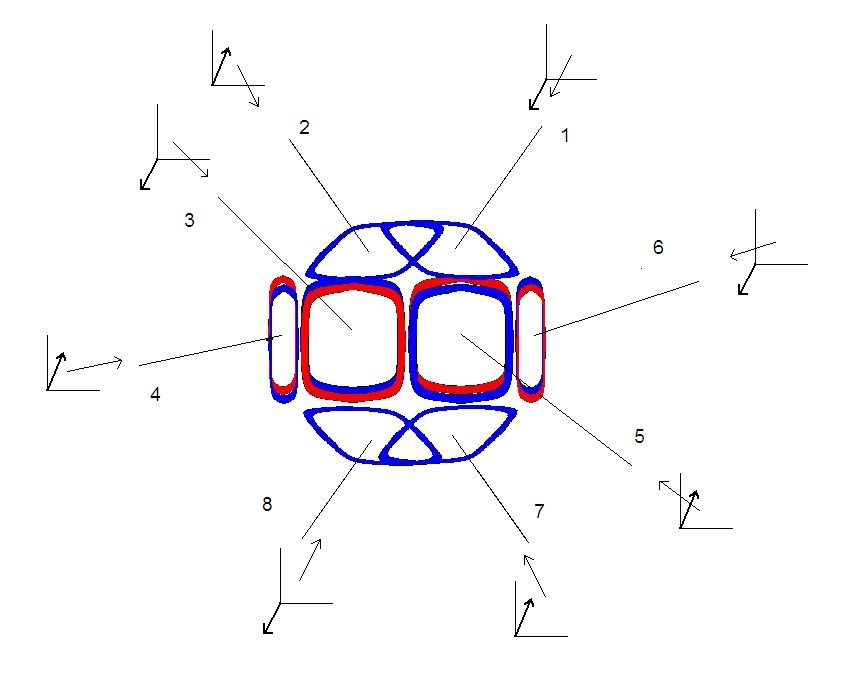
Below is a simplified picture of an Oxygen atom. Protons and neutrons are represented as either a red(neutron) or blue(proton) square. On the top and bottom, only the protons are shown. Extending from each proton is a line representing the direction of the proton's electron. With each line, is a small x, y, x axis to show the direction of the electron toward the nucleus. Either toward you or away from you. On the number 1 electron, the z axis arrow is pointing down, toward you. The numbering scheme is present to designate each electron so it can be easily identified in discussing the angles that form around the Oxygen nucleus.
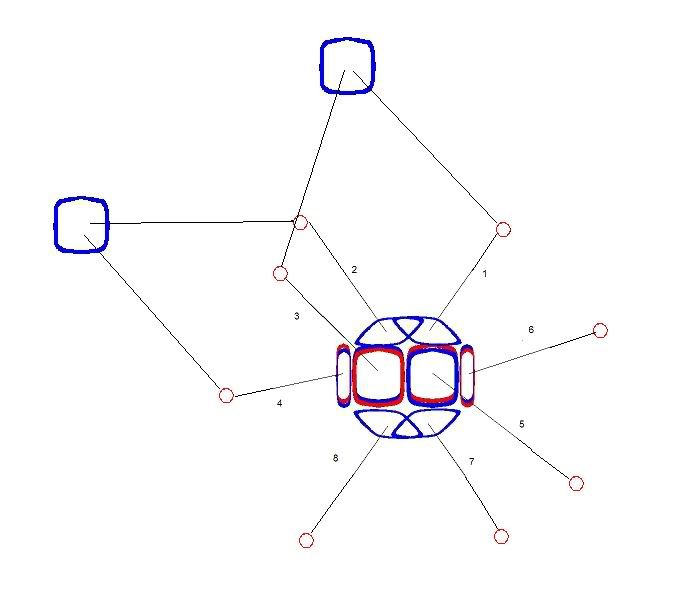
The next image is a simplified picture of a water molecule. The two single blue squares are Hydrogen nuclei -- single protons. Each shares two electrons with the Oxygen atom in covalent bonds. The electrons are single negative Hawkrings. One bonded to the 1 and 3 electrons. The other to the 2 and 4 electrons.
In liquid or gas form, an Oxygen atom shares two sets of its eight electrons with two hydrogen nuclei in covalent bonds. Where each Hydrogen nucleus shares two electrons with the Oxygen nucleus. These two bonds form the well known angle between the two hydrogen atoms in a water molecule, which is about 104.4 degrees. The natural angle between the 1, 3 covalent bond and the 2, 4 covalent bond, if the formation was perfect, would be 105 degrees. But as described earlier, the angle is very slightly changed due to the imperfect neutron formation on the top and bottom of the Oxygen nucleus.
So, the covalent bonds between Oxygen and Hydrogen in liquid water give water molecules the shape seen above, which makes the molecule electrically polar. The leads to the formation of what is called the Hydrogen bond. Causing water molecules to stick to each other. The Hydrogen end of the molecule is electrically positive and the two Hydrogen nuclei form a wedge at 104.5 degrees. When this wedge is near another water molecule, it is attracted to the negative end of the other molecule, the end on the opposite side of that molecule's Hydrogen end. The positively charged wedge shares electric energy with the electrons on the negative side of the other water molecule when the molecules come into contact. The Hydrogen bond is more easily broken and reformed than the covalent bonds that hold each molecule together. This allows liquid water molecules to move around each other easily, yet they are still strongly attracted or bound to each other.
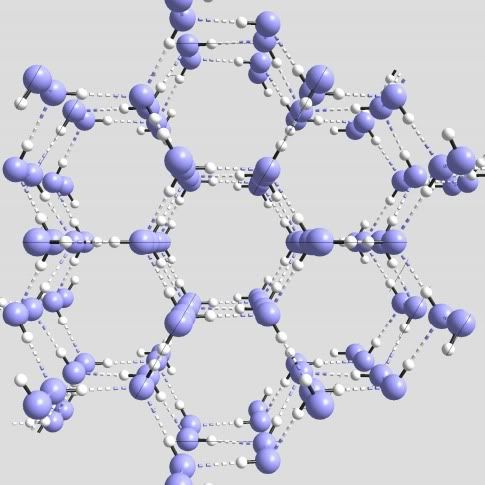
Now I'lll describe the formation of snowflake crystals. And here I'll depart slightly from the way our science describes this formation. As far as I've been able to understand the current thinking on this formation, the bonds between water molecules in snowflakes, remain as they are in liquid water, they just become more static. That is, the covalent bonds in individual molecules remain intact. As do the Hydrogen bonds with other molecules. The only difference is that the molecules become arranged in a hexagonal pattern and stay locked in it while the snowflake is frozen. But I can't find any explanation of why these bonds form the 120 degree angle required for the snowflake crystal formation. If anyone reading does understand the reasoning behind this, I'd appreciate hearing about it in comments.
In my model, the bonds between molecules change as water turns to ice. Instead of a mixture between covalent and Hydrogen bonds, All the bonds are converted to univalent bonds. Where only one electron is shared between each Oxygen and Hydrogen nucleus. Just like in the liquid formation, each Oxygen nucleus bonds with Hydrogen nuclei among 4 of its electrons. But, two of the covalent bonds in the liquid formation become univalent with one of the electrons it was bound to as a liquid. And two new univalent bonds are formed with two other freezing molecules.
Let's look at a picture to see how this would happen. Below are a few water molecules in the liquid state.
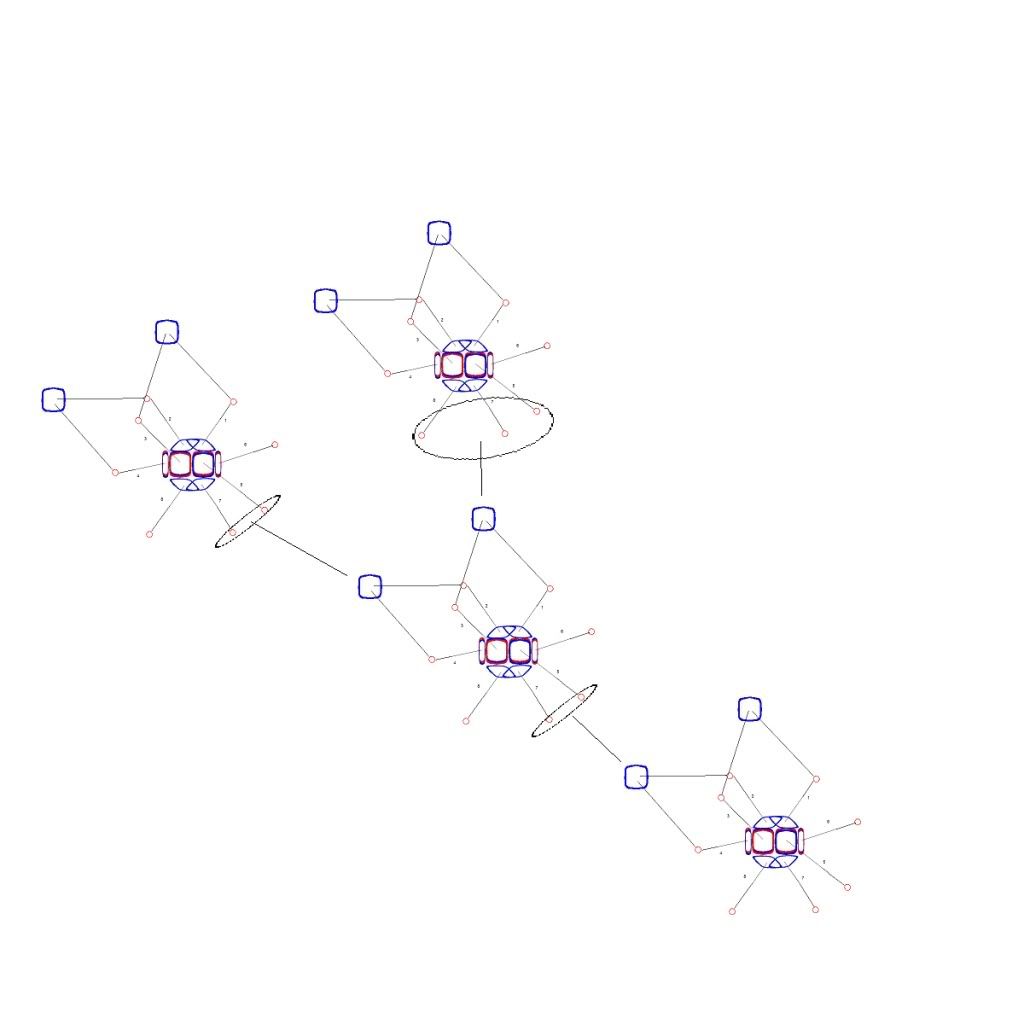
All the Oxygen nuclei are covalently bonded to their two Hydrogen nuclei. The upper two molecules are Hydrogen bonded to the middle molecule. The middle and lower molecules are also Hydrogen bonded. The covalent bonds are between the 1, 3 electrons and the 2, 4 electrons for all the molecules. As the molecules freeze, the 1, 3 bond in the middle molecule becomes univalent with the upper one. Now it is bound to that molecule with just the number 1 electron. The 2, 4 bond is similarly reduced to the number 4 electron, which opposes the number 1 electron at 120 degrees. The number 7 electron's Hydrogen bond also becomes univalent. The 7 electron opposes both the 1 and 4 electrons at 120 degrees -- they are all in one plane, dividing 360 degrees into 3 120 degree angles -- this is the source of the hexagonal snowflake formation. The number 7 electron had been Hydrogen bonded to the middle molecule. Now that bond becomes univalent with the number 7 electron and we have 3 Oxygen molecules rigidly bound in a plane at 120 degrees to each other. One more univalent bond forms in the middle molecule, with either the 3 or 5 electron. This bond is not at 120 degrees to any of the other bonded electrons. It is at roughly 80 degrees to the plane those electrons are in. This bond is what connects the layers of hexagonal formations in the snowflake structure.
In a single hexagonal snowflake ring, each of the six molecules will have two electrons bonded to two others in the ring. And they will have one more electron bonded to other molecules forming other hexagons in the same plane. Each of the six will also have one more bond to a molecule in a ring above or below it. Three of the six will be bonded above and 3 will be bonded below. The up or down orientation of these bonds in a single ring alternates as you go around the ring. This crystal snowflake lattice formation results from the nearly perfect symmetrical geometry of the Oxygen nucleus.
In regular ice, like you have in your freezer -- hexagonal type 1, the formation of water molecules is not as open as in snow. It's more tightly packed, as if you folded a flat hexagon in a weird way. And joined those together in a tighter formation. This structure is more dense and stronger than a snowflake structure. It's strength comes from a simple, sound architectural formation, based on the angles of the Oxygen nucleus. It also comes from the change to univalent bonds that occurs when water freezes. When these bonds are formed, water molecules are pushed out of the way so the structure has room to form. And the crystalized structure of all ice is more open than the arrangement of liquid water. This is why water has the unusual property of expanding by about 10 percent when it freezes. This increase in the volume of water begins to take place when the temperature of water drops below 4 degrees Celsius. And is complete at 0 degrees. As this bond shifting occurs, the volume of water increases as the bonded molecules become rigidly locked in crystal formations.
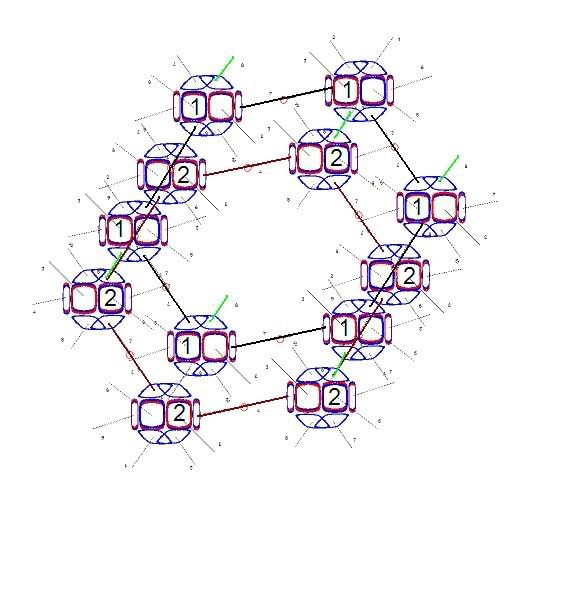
In snowflake crystals, the Hydrogens are bonded to the number 1 and 4 electrons. These two are at almost exactly 120 degrees from each other. and the number 7 electron opposes both of those at the same angle. This electron becomes the focus of another bond. This results in the hexagonal lattice structure in snowflakes, pictured below. Some of the Oxygen nuclei are turned upside down to make the angles come out right. And all the bonds are shown as single covalent bonds between oxygen atoms for simplicity. In an actual snowflake hexagon, Hydrogen atoms would be between the bonds. The purpose of this diagram is to show that the angles in an Oxygen nucleus are the source of the hexagonal lattice crystal structure in snowflakes.
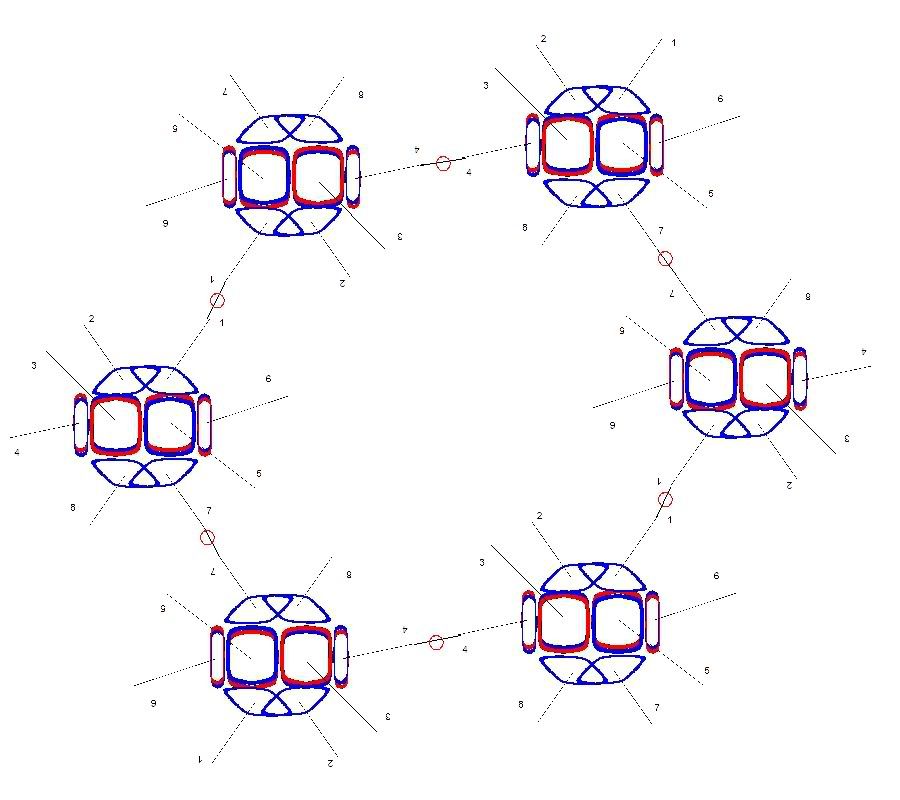
These hexagon structures also form layers one on top of the other. If you connected another water molecule to the number 5 electron in the leftmost Oxygen nucleus above, and also every alternating nucleus in the circle, they would all join to form another hexagon behind this one. Same thing with the number 5 electron beginning a formation of another hexagon in front of this one.
This idea is also picture below in a diagram borrowed from a website at the following Url. http://www.cs.unm.edu/...
And here is a picture of a dendrite snowflake, borrowed from: http://www.its.caltech.edu/...

In the model I've begun to describe here, each element has a specific three dimensional configuration that produces the arrangement of electrons around its nucleus. The arrangement of the electrons and their bonding tendencies and angles are easily seen in the nuclear configurations. And of the 20 or so elements I have figured out so far, the properties all match with those already determined by science. Other effects, such as magnetism, electric and thermal conductivity and density are also explained by the configurations.
I'm currently working out the angles and bonds in the other organic elements: Carbon, Nitrogen, Phosphorus and Sulfur. I plan to have another diary up soon describing the bonds in some organic molecules, including DNA.
I also have several diaries describing the whole theory behind this nuclear model. Including a more thorough description of the mechanics of the nuclear aspect of it. Anyone interested can find them by searching for diaries by Sneelock, or the tags: Einstein, Physics, Chemistry and Science. I would recommend reading them in the following order.
Relativity
New Science
On the Machinery of Motion
Feynman Force
http://sjsu.rudyrucker.com/...
Like the 120 degree angle formed regularly in snowflakes, liquid or gaseous water has a consistent angular formation. With the hydrogen atoms always sharing two sets of two electrons on one side of the Oxgen nucleus. And the two Hydrogen nuclei always forming an angle of about 104.4 degrees with each other.
As I mentioned before, The geometry of the various angles in which the elements arrange themselves is not yet fully understood. There are theories about how the angles result from the attractive and repulsive forces of the electrons in atoms. But they don't work consistently across different elements. But we do have descriptions of what the angles are for most elements. And we know which elements can combine with each other. As well as the angles that form between them when they do. But we haven't understood in a convincing way, why the elements form the angles that they do. We don't have anything that explains the source of the geometry. We just know what the geometry is.
Below is a model of nuclear formation that goes beyond the current one. A model that describes the three dimensional configuration of protons and neutrons in a nucleus -- and accounts for the resulting geometry of atomic bonds that science has already determined. Oxygen is just one example of an element that this model explains the geometry of, more convincingly than the current model. I'll give a brief description of the model, then focus on the resulting configuration for Oxygen, which determines the known bonding angles that form in both water and snowflakes.
In this model, protons and neutrons are composed of different numbers of the same elements -- called Hawkrings. There are two types of Hawkrings. Left(positive) rings and right(negative) rings. A neutron consists of 4 left Hawkrings and 4 right Hawkrings, joined together in an alternating chain of 8 rings. The chain joins end to end forming a circle of 8 rings. The rings are bound together by gluons. Below is a picture of a neutron in its natural state, a circle. The shape it forms, if it's not attached to another hadron. I'll use the term hadron here to refer to either protons or neutrons, or both -- though this term generally refers to anything inside a nucleus.

A proton is formed from the same configuration as a neutron, except that an additional left ring joins the formation in the center, bonding to the 4 right rings in the outer ring. This gives a proton a square shape, pictured below.

Neutrons are more flexible than protons. They can assume the square shape of a proton, when they are bound to other hadrons, or they can accommodate themselves to other shapes. In the spherical configuration of an Oxygen nucleus, neutrons do both of these things. A squared proton is below.

In the formation of the elements, individual Hawkrings in protons and neutrons become bonded to rings in other hadrons. By gluons, that join these building blocks together in certain shapes. The four Elements following Hydrogen, Helium(2), Lithium(3), Beryllium(4), and Boron(5), all have box-like shapes. But the next 5, Carbon(6), Oxygen(8), Nitrogen(9), Fluorine(10) and Neon(11) are all spheres. Carbon is built on a six-sided ring, with 6 more hadrons filling out the top and bottom. And the others all built on a similar eight-sided ring, with differing numbers of protons and neutrons above and below, according to their atomic number.
The formation of an Oxygen nucleus is pictured below. First an alternating ring of 4 protons and 4 neutrons forms a ring. On top, two protons attach to two opposing neutrons in the ring. Then two neutrons attach to the ring and to the corners of the protons. Tying the top together. And leaving the two protons facing up at almost exactly 45 degrees from horizontal. Perhaps a little bit higher than that because the neutrons are not attached to the ring as symmetrically as the protons. The bottom is the same configuration, but rotated 90 degrees relative to the top.

Oxygen(8) is the most perfectly symmetrical of all the spheres. And it's easy to see how the geometry of the Oxygen nucleus creates the amazing properties of water. The location of the proton faces in a nucleus determines where an element's electrons will be arranged. In Oxygen, The 8-ring of alternating protons and neutrons means, if you were standing in the center of the nucleus, directly in front, behind and to your right and left, there would be a proton, and an electron extending directly out from each of those. Above, would be two more protons and electrons. If you turn 45 degrees left and raise your arms up 45 degrees above horizontal, that's where the two top electrons will be. Same for the bottom, but you'd rotate 45 degrees right from your original position. So the top and bottom proton faces are oriented at 90 degrees to one another. The angles formed by these eight lines fit perfectly with what science has determined about the bonding angles in water and in snowflakes.
Below is a simplified picture of an Oxygen atom. Protons and neutrons are represented as either a red(neutron) or blue(proton) square. On the top and bottom, only the protons are shown. Extending from each proton is a line representing the direction of the proton's electron. With each line, is a small x, y, x axis to show the direction of the electron toward the nucleus. Either toward you or away from you. On the number 1 electron, the z axis arrow is pointing down, toward you. The numbering scheme is present to designate each electron so it can be easily identified in discussing the angles that form around the Oxygen nucleus.

The next image is a simplified picture of a water molecule. The two single blue squares are Hydrogen nuclei -- single protons. Each shares two electrons with the Oxygen atom in covalent bonds. The electrons are single negative Hawkrings. One bonded to the 1 and 3 electrons. The other to the 2 and 4 electrons.

In liquid or gas form, an Oxygen atom shares two sets of its eight electrons with two hydrogen nuclei in covalent bonds. Where each Hydrogen nucleus shares two electrons with the Oxygen nucleus. These two bonds form the well known angle between the two hydrogen atoms in a water molecule, which is about 104.4 degrees. The natural angle between the 1, 3 covalent bond and the 2, 4 covalent bond, if the formation was perfect, would be 105 degrees. But as described earlier, the angle is very slightly changed due to the imperfect neutron formation on the top and bottom of the Oxygen nucleus.
So, the covalent bonds between Oxygen and Hydrogen in liquid water give water molecules the shape seen above, which makes the molecule electrically polar. The leads to the formation of what is called the Hydrogen bond. Causing water molecules to stick to each other. The Hydrogen end of the molecule is electrically positive and the two Hydrogen nuclei form a wedge at 104.5 degrees. When this wedge is near another water molecule, it is attracted to the negative end of the other molecule, the end on the opposite side of that molecule's Hydrogen end. The positively charged wedge shares electric energy with the electrons on the negative side of the other water molecule when the molecules come into contact. The Hydrogen bond is more easily broken and reformed than the covalent bonds that hold each molecule together. This allows liquid water molecules to move around each other easily, yet they are still strongly attracted or bound to each other.
Now I'lll describe the formation of snowflake crystals. And here I'll depart slightly from the way our science describes this formation. As far as I've been able to understand the current thinking on this formation, the bonds between water molecules in snowflakes, remain as they are in liquid water, they just become more static. That is, the covalent bonds in individual molecules remain intact. As do the Hydrogen bonds with other molecules. The only difference is that the molecules become arranged in a hexagonal pattern and stay locked in it while the snowflake is frozen. But I can't find any explanation of why these bonds form the 120 degree angle required for the snowflake crystal formation. If anyone reading does understand the reasoning behind this, I'd appreciate hearing about it in comments.
In my model, the bonds between molecules change as water turns to ice. Instead of a mixture between covalent and Hydrogen bonds, All the bonds are converted to univalent bonds. Where only one electron is shared between each Oxygen and Hydrogen nucleus. Just like in the liquid formation, each Oxygen nucleus bonds with Hydrogen nuclei among 4 of its electrons. But, two of the covalent bonds in the liquid formation become univalent with one of the electrons it was bound to as a liquid. And two new univalent bonds are formed with two other freezing molecules.
Let's look at a picture to see how this would happen. Below are a few water molecules in the liquid state.

All the Oxygen nuclei are covalently bonded to their two Hydrogen nuclei. The upper two molecules are Hydrogen bonded to the middle molecule. The middle and lower molecules are also Hydrogen bonded. The covalent bonds are between the 1, 3 electrons and the 2, 4 electrons for all the molecules. As the molecules freeze, the 1, 3 bond in the middle molecule becomes univalent with the upper one. Now it is bound to that molecule with just the number 1 electron. The 2, 4 bond is similarly reduced to the number 4 electron, which opposes the number 1 electron at 120 degrees. The number 7 electron's Hydrogen bond also becomes univalent. The 7 electron opposes both the 1 and 4 electrons at 120 degrees -- they are all in one plane, dividing 360 degrees into 3 120 degree angles -- this is the source of the hexagonal snowflake formation. The number 7 electron had been Hydrogen bonded to the middle molecule. Now that bond becomes univalent with the number 7 electron and we have 3 Oxygen molecules rigidly bound in a plane at 120 degrees to each other. One more univalent bond forms in the middle molecule, with either the 3 or 5 electron. This bond is not at 120 degrees to any of the other bonded electrons. It is at roughly 80 degrees to the plane those electrons are in. This bond is what connects the layers of hexagonal formations in the snowflake structure.
In a single hexagonal snowflake ring, each of the six molecules will have two electrons bonded to two others in the ring. And they will have one more electron bonded to other molecules forming other hexagons in the same plane. Each of the six will also have one more bond to a molecule in a ring above or below it. Three of the six will be bonded above and 3 will be bonded below. The up or down orientation of these bonds in a single ring alternates as you go around the ring. This crystal snowflake lattice formation results from the nearly perfect symmetrical geometry of the Oxygen nucleus.
In regular ice, like you have in your freezer -- hexagonal type 1, the formation of water molecules is not as open as in snow. It's more tightly packed, as if you folded a flat hexagon in a weird way. And joined those together in a tighter formation. This structure is more dense and stronger than a snowflake structure. It's strength comes from a simple, sound architectural formation, based on the angles of the Oxygen nucleus. It also comes from the change to univalent bonds that occurs when water freezes. When these bonds are formed, water molecules are pushed out of the way so the structure has room to form. And the crystalized structure of all ice is more open than the arrangement of liquid water. This is why water has the unusual property of expanding by about 10 percent when it freezes. This increase in the volume of water begins to take place when the temperature of water drops below 4 degrees Celsius. And is complete at 0 degrees. As this bond shifting occurs, the volume of water increases as the bonded molecules become rigidly locked in crystal formations.
In snowflake crystals, the Hydrogens are bonded to the number 1 and 4 electrons. These two are at almost exactly 120 degrees from each other. and the number 7 electron opposes both of those at the same angle. This electron becomes the focus of another bond. This results in the hexagonal lattice structure in snowflakes, pictured below. Some of the Oxygen nuclei are turned upside down to make the angles come out right. And all the bonds are shown as single covalent bonds between oxygen atoms for simplicity. In an actual snowflake hexagon, Hydrogen atoms would be between the bonds. The purpose of this diagram is to show that the angles in an Oxygen nucleus are the source of the hexagonal lattice crystal structure in snowflakes.

These hexagon structures also form layers one on top of the other. If you connected another water molecule to the number 5 electron in the leftmost Oxygen nucleus above, and also every alternating nucleus in the circle, they would all join to form another hexagon behind this one. Same thing with the number 5 electron beginning a formation of another hexagon in front of this one.

This idea is also picture below in a diagram borrowed from a website at the following Url. http://www.cs.unm.edu/...

And here is a picture of a dendrite snowflake, borrowed from: http://www.its.caltech.edu/...

In the model I've begun to describe here, each element has a specific three dimensional configuration that produces the arrangement of electrons around its nucleus. The arrangement of the electrons and their bonding tendencies and angles are easily seen in the nuclear configurations. And of the 20 or so elements I have figured out so far, the properties all match with those already determined by science. Other effects, such as magnetism, electric and thermal conductivity and density are also explained by the configurations.
I'm currently working out the angles and bonds in the other organic elements: Carbon, Nitrogen, Phosphorus and Sulfur. I plan to have another diary up soon describing the bonds in some organic molecules, including DNA.
I also have several diaries describing the whole theory behind this nuclear model. Including a more thorough description of the mechanics of the nuclear aspect of it. Anyone interested can find them by searching for diaries by Sneelock, or the tags: Einstein, Physics, Chemistry and Science. I would recommend reading them in the following order.
Relativity
New Science
On the Machinery of Motion
Feynman Force
http://sjsu.rudyrucker.com/...


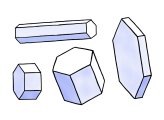 Simple
Simple 


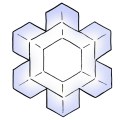 Stellar Plates
Stellar Plates


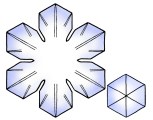 Sectored Plates
Sectored Plates


 Stellar
Stellar 


 Fernlike
Fernlike 


 Hollow Columns
Hollow Columns


 Needles
Needles


 Capped
Capped 



 Double Plates
Double Plates






 Triangular
Triangular 


 12-Sided
12-Sided 


 Bullet
Bullet 


 Radiating
Radiating 

 Rimed
Rimed 



